Time to read: 3 min
“Medical device” is a broad umbrella term that covers a huge variety of apparatus and equipment, such as Band-Aids, dental floss, blood pressure cuffs, defibrillators, MRI scanners, and much, much more. It’s probably no surprise that medical device design constitutes a major segment of the field of mechanical engineering, especially in the U.S., which is a global leader in medical device R&D and consumption.
The development process for a medical device is no different than any other device: Design, prototype, test, and repeat. However, the material requirements are more exacting. Many medical device prototypes need biocompatible and/or sterilizable materials, due to testing and clinical trial requirements.
Biocompatible Materials
For plastics, the most stringent designation is USP Class VI. USP class VI testing involves three in vivo biological reactivity evaluations performed on animals, including:
- Acute Systemic Toxicity (Systemic Injection) Test: This test measures the irritant effect when the sample is administered orally, applied to the skin, and inhaled.
- Intracutaneous Test: This test measures the irritant effect when the sample is in contact with live subdermal tissue.
- Implantation Test: This test measures the irritant effect of an intramuscular implantation of the sample into a test animal over five days.
3D printing can produce virtually unlimited geometries, which is great for rapid iteration of complex designs. Of the 3D printing materials that China Manufacturing parts, Inc. offers, VeroClear and Nylon are USP Class VI certified. In particular, VeroClear is used for quick-turn dental and orthopedic surgical guides.
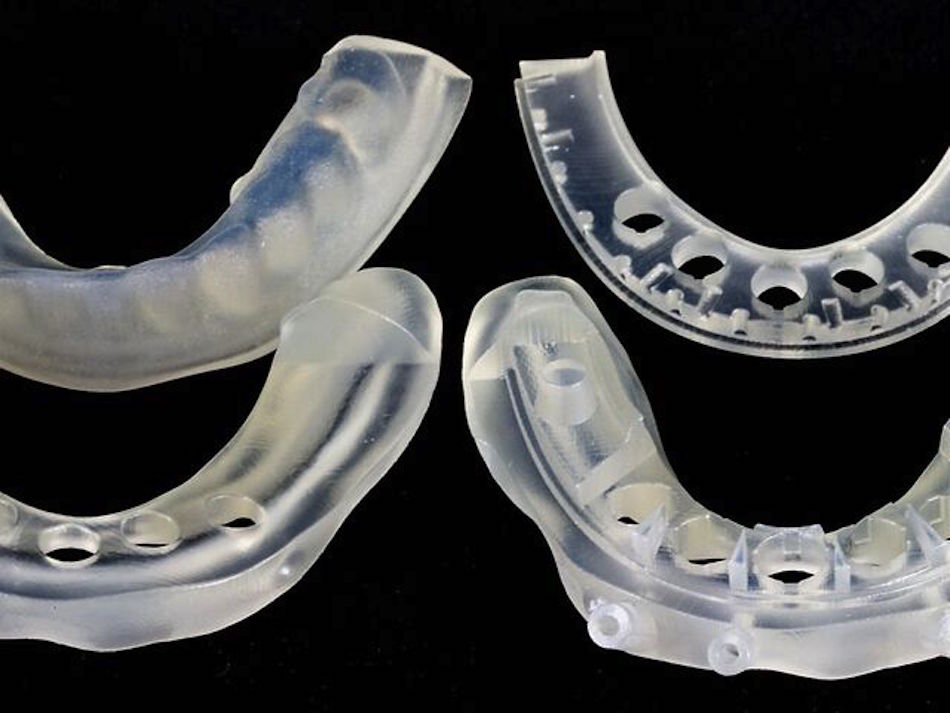
Both VeroClear and Nylon are printed in high resolution, which is often required for small medical device prototypes.
CNC machining is suitable for prototyping and end-use production of medical device parts. There are a lot more material choices, and the materials are more robust. However, the design requires more attention to ensure machinability.
The following materials are available in certified USP Class VI grades:
- Acetal (also known as Delrin or POM)
- Polypropylene (PP)
- Polyetherimide (PEI)
- Polyether ether ketone (PEEK)
- Polysulfone (PSU)
- Polyphenolsulfone (PPSU)
If you’re making earlier stage prototypes that will not be used in experiments or clinical trials, consider using non-certified plastics. You’ll get the same mechanical properties without paying the higher price tag. Delrin 150 is an excellent materials for early stage prototyping.
CNC machining can also produce parts in biocompatible metals. There are three common implant grade choices:
- Stainless Steel 316L
- Titanium Grade 5, also known as Ti6Al4V or Ti 6-4
- Cobalt-Chrome (CoCr)

Stainless Steel 316L is the most commonly available material out of the three. Titanium has better weight-to-strength ratio, but it is significantly more expensive. CoCr is mostly used for orthopedic implants. We recommend that you prototype with SS 316L while the design is being refined, then move onto the more expensive materials when the design is more mature.
Sterilizable Materials
Any re-usable medical devices that might come in contact with blood or bodily fluids must be sterilizable; hence, most medical devices used in healthcare facilities are made of sterilizable materials. There are many methods of sterilization: heat (dry heat or autoclave/steam), pressure, chemicals, irradiation, etc.
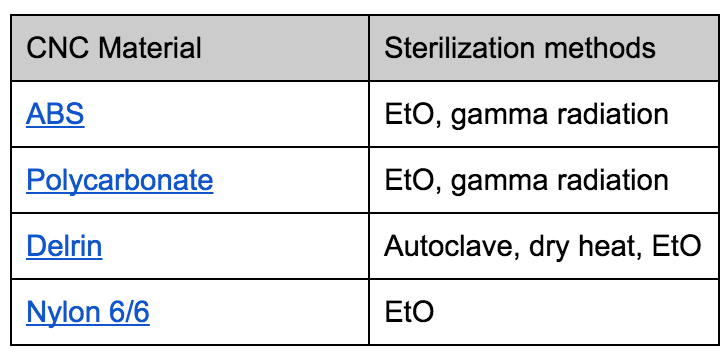
Chemicals and irradiation are the preferred methods of sterilizing plastics, because heat can break down plastics. Here is a chart that outlines many plastics’ compatibility with different sterilization methods. Autoclave and dry heat is more common for sterilizing metals.
All of the USP Class VI certified materials we mentioned previously are sterilizable, as are the implant-grade metals.
In addition, China Manufacturing parts, Inc. offers two 3D printed materials that are sterilizable by Ethylene oxide (EtO) treatment and gamma radiation:
Again, CNC machining provides the most amount of choice for sterilizable materials.
If the medical device is intended for patients to sterilize at home, the material should also be compatible with disinfectant chemicals, such as bleach, ethanol, isopropyl alcohol, iodine, and hydrogen peroxide. ABS and Delrin are the most chemically resistant plastics China Manufacturing parts, Inc. offers.
When to Use Medical Grade Materials
Definitely use medical grade materials when you are building prototypes for experiments or clinical trials. However, for early form and fit prototypes, using generic materials can save you a lot of money.

