Time to read: 5 min
We all know what happens when metal is exposed to the elements: rust. When designing and creating your products, the last thing you want to discover is an uneven and grungy layer of corrosion.
Although this is an age old problem, there are many ways to circumvent it. One of the best solutions to avoiding corrosive wear is to coat your part, thereby protecting it from the natural elements that cause the degradation.
Coatings take many forms, each delivering their own unique properties. This article covers some of the most common kinds of conversion coatings.
Conversion coatings, unlike passive coatings like paint or a powder coat, involve a chemical reaction at the surface of your metal part. Although this process can be applied to almost all metals, this article will more specifically outline the pros and cons of a few broad categories of conversion coating: passivation and black oxide, which are used for commonly used for coating steel and stainless steel, as well as alodine and anodizing, which are most commonly used on aluminum parts.
What is Conversion Coating?
A conversion coating is a metal oxide layer on the surface of a metal part that protects it from corrosion, rust, and other wear, and that can provide useful material properties. Because most metals are naturally reactive to oxygen, even when not coated, metal oxides form at the surface of metal parts. Unaltered, these naturally forming chemical deposits are often uneven and not aesthetic. We are all familiar with the classic red rust that tarnishes old fences and tractors left out in the field.
However, if these metal oxides are created in a controlled environment, the surface reactions can instead be harnessed to protect metal parts by creating an inert surface impenetrable to uneven corrosion. Conversion coatings use special techniques like acidic baths or electricity to drive the formation of a metal oxide crystal structure that shields the metal part from external oxygen and other corrosive elements.
Nanopores within the crystal layer can often be filled with dye or other sealants in order to achieve enhanced properties like color, lubricity and increased hardness. Most importantly, the metal oxide layer is chemically bonded to the metal which it is protecting. This makes conversion coating an extremely durable option.
How is the conversion coating different from plating?
Plating and conversion coatings seem similar. They both use baths and electrolysis to form a protective layer on the surface of a metal part. However, in the plating process, there is no chemical reaction on the surface of the part. Rather, the metal is covered by another metal. This covering, although typically quite strong, can wear in a similar fashion to paint.
Finishes for Aluminum
Anodizing
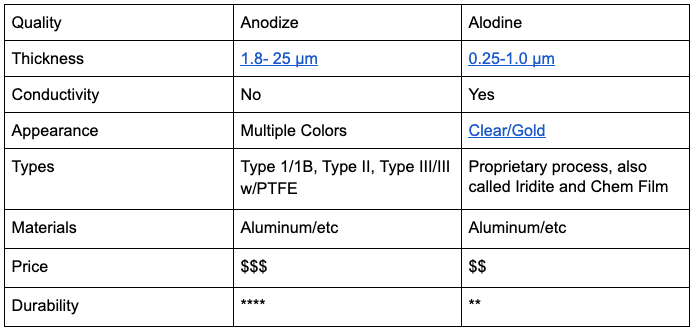
Anodizing is a common form of electrolytic passivation for aluminum. After stripping away any pre-formed corrosion in a sulfuric acid bath, parts are positively charged by an electric current. Parts then attract negatively charged, and very reactive oxygen atoms directly to their surface. The metal oxide crystal nanopores formed in this process can be filled with dye revealing a wide range of colors before being sealed in another chemical bath. Because of the brilliant spectrum of color available through the anodizing process, it is a common coating for consumer products.

Anodizing adds a non-significant amount of material to the surface of the part in order to cause a reaction, therefore, the parts grow outward. Any holes in the parts will become smaller, so it is important to be aware of any tight tolerances and common to plug or cover these areas before anodizing. Different anodizing processes (Type 1/1B, Type II, Type III/III w/PTFE) result in different layer thicknesses. These different types of anodizing allow this coating to be useful in a range of industries and applications, from trusses on the NASA space station to artistic sculpture and sporting goods.
Alodine
Alodine, also called chem film or Iridite, is similar to anodizing but does not use electricity in the creation of its oxidative layer. Instead, a very reactive chromium atom is used to drive the purely chemical reaction. Historically, a hexavalent chromium compound was the main catalyst in alodine conversion coatings; however, after discovering this atom’s carcinogenic nature, it has become tightly regulated and replaced with a trivalent chromium atom.
Parts are placed in the chromium bath, rinsed, and then left to dry. The coating starts out squishy and gel-like, but as the chemical reaction continues, the coating hardens into a conductive and protective layer. Alodine is often applied to screws, bolts, brackets and other fasteners because it does not produce a noticeable difference in dimension, but can be used in many other industries as well. Alodine is cheaper than anodizing, but more susceptible to wear and scratches.

Can I Use Both Anodizing and Alodine?
Yes! It is quite common to apply both coatings, especially for parts where material properties need to vary throughout the part surface. For example, if a design requires specific tolerances on certain edges or holes, an alodine finish can be applied first to protect the entire part.
To provide extra durability, an anodized coating can follow where tolerances do not matter quite as much. In addition, some cnc machining aluminum parts require only specific sections of their surface to be conductive. In this case, one can selectively apply alodine and anodized finishes to achieve appropriate electrical properties.
Steels/Stainless Steels
Passivation
Although the term passivation is often used as a general term for conversion coating, there are a few passivation processes that are commonly used for coating steel and stainless steel parts. Stainless steel has been optimized for its anti-corrosion properties. Its high chromium content makes passivation simple. Once parts are cleaned, the alloyed chromium near the part surface reacts with oxygen, and a protective barrier is formed.
Another common passivation technique for steels and stainless steels is a phosphate conversion coating. This coating process is driven by phosphoric acid and phosphate salts which are applied to the metal part and react to create stable, metalophosphate nanopores ready for either a manganese or zinc seal (also called parkerization).

Black Oxide
Black oxide, historically called browning or bluing, is a conversion coating most often used for steel and stainless steel that is very similar to the alodine process for aluminum. Over many millennia of existence, a variety of procedures have been developed for applying black oxide finish. Ultimately, it is a purely chemical process which results in a conductive iron oxide layer called magnetite.
The black oxide finish greatly decreases reflection from the metal’s surface and barely changes the dimensions of the coated part. When a black oxide finish is sealed with oil or wax, metal parts notice an increase in lubricity and are therefore used often to prevent galling, like in gears or locks.


Can I Use Both Passivation and Black Oxide?
Yes! In fact, this is commonly done. The black oxide finish creates a surface that works well with additional passivation finishes which can greatly increase the longevity and corrosion-resistant properties of metal products.
Ultimately, applying a conversion coating to your parts can help them last longer and can add desired properties like color, hardness, lubricity, or increased conductivity. Therefore, it’s important to keep in mind these common conversion coatings when designing and optimizing your next product!

