Time to read: 4 min
Prototyping for medical devices and applications produces functional samples for testing and evaluation. These samples can be used to advance a product through regulatory gates, or approval stages, but that’s not the only reason why medical prototyping is necessary. Designers can apply what they learn and make changes, including improvements for manufacturability. When prototyping is complete, low-volume production also provides an opportunity to refine not just the design, but also the manufacturing process.

For high-volume production, medical injection molding offers quality, consistency, and support for various thermoplastic materials. Yet, the tooling that’s used can be expensive, at least when the cost of the injection mold is only spread across a small number of parts produced with it. Medical designers can use 3D printing for prototyping and low-volume production instead, but with some important limitations. Plus, there are ways to use plastic injection molding to produce cost-effective medical prototypes and lower volumes of parts.
Medical Prototypes: 3D Printing and Injection Molding
Medical injection molding and 3D printing are not mutually exclusive. In other words, there’s a role for both if you understand when to use them and for which types of prototypes. For example, alpha prototypes are essentially non-functional models. Often, medical manufacturers 3D print them so that they can see product dimensions without paying for or waiting for tooling. Beta prototypes can also be 3D printed to advance a design; however, these prototypes don’t represent the final product.

Pilot prototypes are produced to engineering specifications and can be used for clinical trials and soft launches. They’re an important milestone in the development process because they use the same material as the final product. If you’re seeking regulatory approval for a medical device, using anything but the end-use material won’t advance your project beyond the initial gates. Even when 3D printers use the same plastics as injection molding machines, 3D printed plastics don’t have identical end-use properties.
Advantages of Injection Molded Medical Prototypes
With injection-molded medical prototypes, you’ll do more than pass through additional regulatory gates. You can still adjust your product design and calibrate dimensions and tolerances, and you can resolve design for manufacturing (DFM) challenges before high-volume manufacturing begins. That can save you time and money when it’s time to fully launch your product, and you’ll be ready to scale-up production since you’re already using medical injection molding instead of some other technology.
Choosing the right partner is important, however. For example, an injection molder who uses mold simulation and analysis software can produce higher quality parts with optimized cycle times and fewer tooling trials. From simulating the fill and packing phases to optimizing gate locations and sizes to improving specific process conditions, a skilled injection molder can minimize part defects. Before high-volume production begins, you’ll be ready to avoid common problems like sink marks, flash, and warping.
Tooling for Medical Prototyping and Low-Volume Production
Medical injection molding for prototyping and low-volume production has its advantages, but how can you justify the cost of tooling? After all, some injection molds can cost thousands or even tens of thousands of dollars. China Manufacturing parts, Inc. can produce tooling cost-effectively, however, and from either aluminum or steel. Both mold materials can be used with a master die unit and frame that includes sprues and runners, leader pins, water lines, and ejector pins. With this approach, you pay for only the mold cavity and the core details.
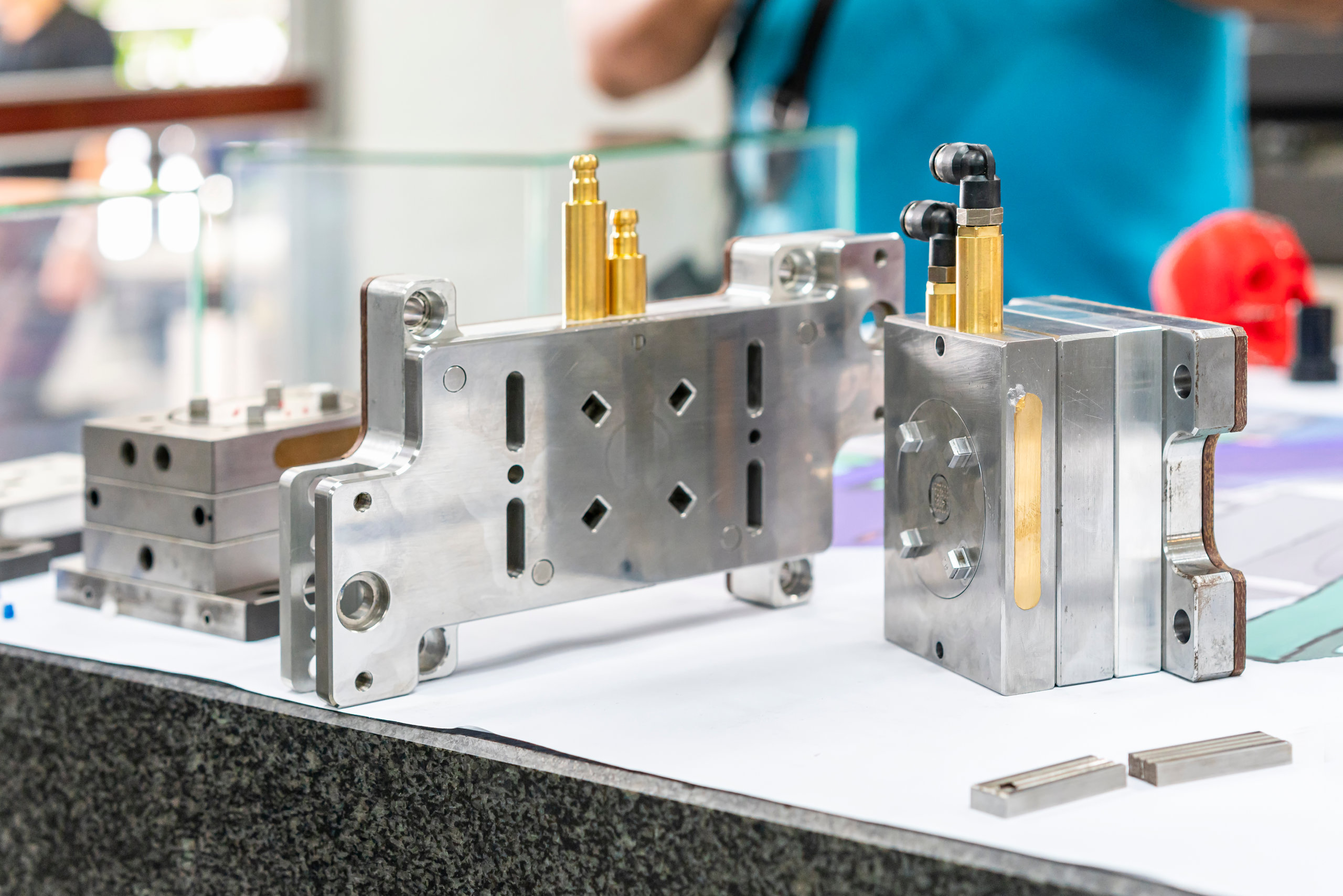
Family molds also provide a way to control tooling costs during medical prototyping and low-volume production. Because family molds contain more than one cavity, you can reduce tooling costs by having multiple different designs inside the same mold. Yet, a family mold can also contain cavities for different iterations of the same part instead. That’s useful during prototyping so that a designer can compare different versions of the same part.
Additional Considerations: Validation, Verification, and Variation
Medical injection molding for prototyping and low-volume production also supports process validation and process verification, both of which are important for medical device manufacturing. To reduce the risk that a part isn’t functional or won’t meet specifications, validation starts before production ever begins. An injection molder reviews your designs, establishes process parameters, and monitors test and production data. Unlike validation, process verification happens after molding is complete.

Finally, medical injection molding for prototyping and low-volume production supports the analysis of defects in the form of part variations. Injection molders that use statistical process control (SPC) can collect and analyze quality data in the form of measurements and instrument readings. Process capability measurements can capture variances and help medical injection molders predict the long-term performance of a process.
Medical injection molding isn’t the only option for prototyping and low-volume production; however, this proven technology can help you to move through gates more quickly while improving designs and controlling costs. At China Manufacturing parts, we have the resources, know-how, and manufacturing network to deliver the plastic injection molding products that you need. Learn more about our injection molding services and create an account to get a quote today!

